The ethical dilemma of gene editing: Our reporter took your questions
By Carolyn Y. Johnson,
The Washington Post [cites CGS’ Pete Shanks ]
| 12. 08. 2023
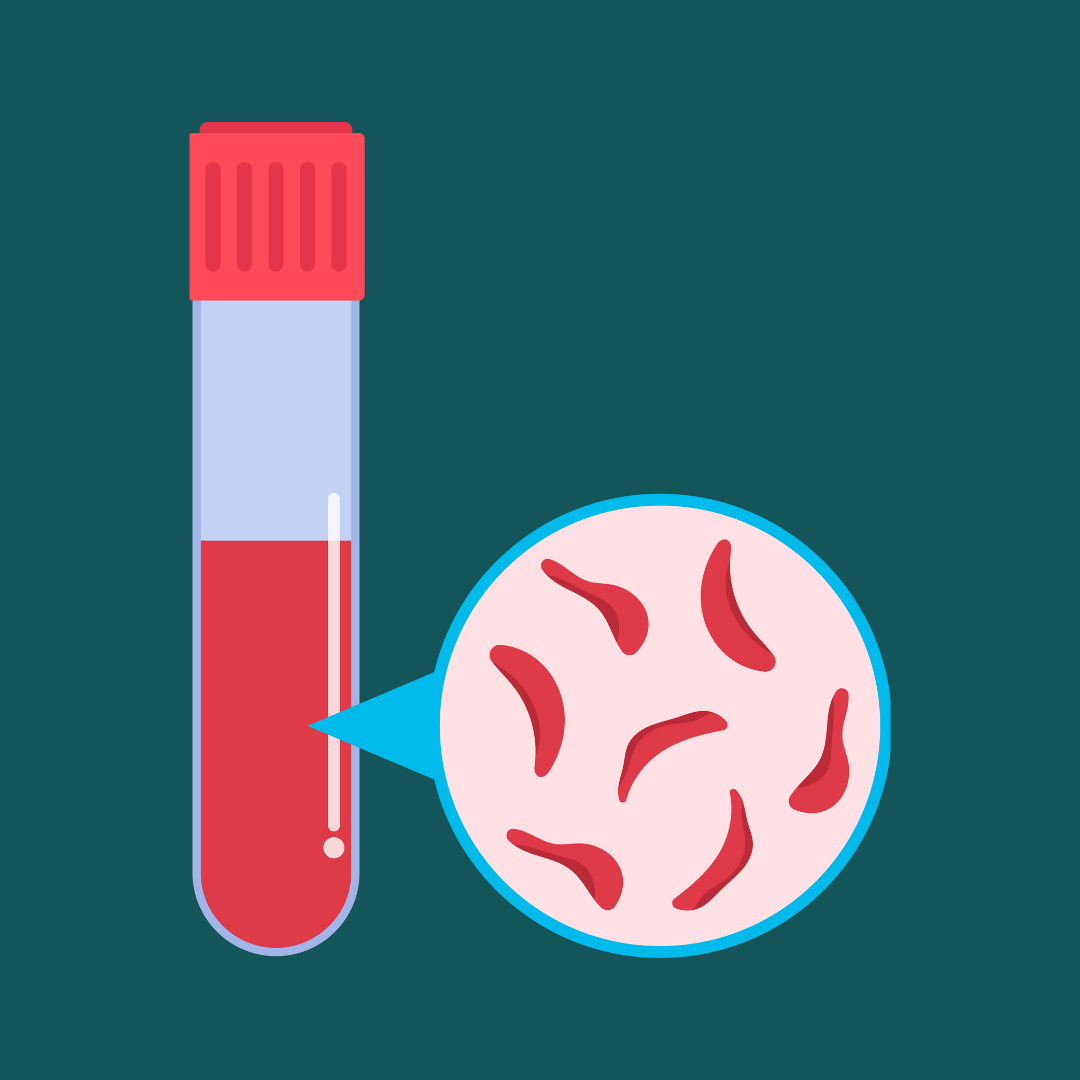
The first medicine based on gene editing, a one-time therapy for sickle cell disease, was just approved in the United States. It’s a big moment for patients with sickle cell disease and for the technology called CRISPR, which powers the therapy.
I am Carolyn Johnson, a science reporter at The Washington Post, and I’ve been following the speedy trajectory of CRISPR from a scientific breakthrough in 2012 to a medicine that can alleviate human suffering 11 years later. On Tuesday, I answered your questions about the potential of this technology to transform medicine — and the challenges associated with it.
First, a quick primer: CRISPR is often compared to a pair of “molecular scissors” that can make targeted cuts in DNA, giving scientists the ability to easily and precisely alter the genome. Scientists often hear from families afflicted by genetic diseases hoping that this technology will help save their loved ones. It has also spurred controversies — including battles over who invented CRISPR and the fear that it will be used to create “designer babies.”
Here...
Related Articles
By Scott Solomon, The MIT Press Reader | 02.12.2026
Chris Mason is a man in a hurry.
“Sometimes walking from the subway to the lab takes too long, so I’ll start running,” he told me over breakfast at a bistro near his home in Brooklyn on a crisp...
By Diaa Hadid and Shweta Desai, NPR | 01.29.2026
MUMBRA, India — The afternoon sun shines on the woman in a commuter-town café, highlighting her almond-shaped eyes and pale skin, a look often sought after by couples who need an egg to have a baby.
"I have good eggs,"...
By George Janes, BioNews | 01.12.2026
A heart attack patient has become the first person to be treated in a clinical trial of an experimental gene therapy, which aims to strengthen blood vessels after coronary bypass surgery.
Coronary artery bypass surgery is performed to treat...
By Staff, ScienceDaily | 01.05.2026
Scientists at UNSW Sydney have developed a new form of CRISPR technology that could make gene therapy safer while also resolving a decades-long debate about how genes are switched off. The research shows that small chemical markers attached to DNA
...