News
Pronatalism is an old idea with roots in eugenics and nationalism, that is now fashionable among far-right influencers and policymakers. They talk...
This is the 10th installment in the Legacies of Eugenics series, which features essays by leading thinkers devoted to exploring...
"In a country nervous about genetically modified crops, we are making the foolhardy move to genetically modified babies." So said...
According to the KMSP report for which I was interviewed, in 2007 a psychiatric research team led by...
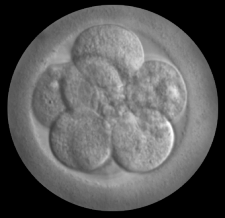
The UK Human Fertilisation and Embryology Authority (HFEA) today released its update on the safety and efficacy of “three-person IVF” (often misleadingly called “mitochondrial replacement”). The HFEA panel’s strongest statement of endorsement is that “the evidence it has seen does not suggest that these techniques are unsafe.” It also acknowledges that “there are still experiments that need to be completed before clinical treatment should be offered.”
“These are tepid conclusions that leave critical safety and efficacy questions unresolved,” said Marcy Darnovsky, PhD, Executive Director of the Center for Genetics and Society. “Much of what the HFEA has described as essential research has not yet been conducted,” she continued. “Some of the research it previously said was critical – for example, success in a non-human primate model – has simply been dropped.”
The UK government has said that it may soon bring this issue to Parliament. After reviewing the HFEA report, Darnovsky said that “the lack of progress makes it clear that a Parliamentary vote would be premature. It certainly provides no incentive to consider changing UK law in order to bring currently illegal techniques into fertility clinics.”
Scientists not involved with developing these techniques have raised questions about whether they could introduce new abnormalities in future children, due to the cellular manipulations, the reagents used, or nuclear-mitochondrial incompatibilities. In addition, it is unclear whether they would be effective in preventing transmission of mitochondrial diseases.
“These are biologically extreme and risky procedures,” Darnovsky said, “especially in light of the available safer alternatives of using pre-implantation genetic diagnosis or eggs provided by other women.”
The HFEA report lists 14 statements and letters that it received in response to its “call for evidence.” One is from Shoukrat Mitalipov, an Oregon researcher who has filed a patent application for one of the techniques under consideration, and set up a company to commercialize it. All the remaining communications appear to raise critical concerns about “three-person IVF” techniques. A letter submitted by the Center for Genetics and Society was signed by 53 prominent scholars, scientists and advocates from around the world.
“Unfortunately, the HFEA’s report does not provide any substantive information about these communications, nor does it make them publicly available. In effect, it has buried this resource,” Darnovsky commented.
A decision to approve clinical use of “three-person IVF” would make the UK the world’s only jurisdiction to allow inheritable genetic modification. “Dozens of countries have drawn a clear policy line that can keep the door shut on manipulating the genes of future children and generations,” Darnovsky said. “We urge the UK government, the public, and Members of Parliament not to let enthusiasm for novel technologies trump the need for clear evidence and for serious consideration about the policy implications of a decision on these controversial techniques.”
Contact:
Marcy Darnovsky
1-510-625-0819 x305
mdarnovsky[AT]geneticsandsociety[DOT]org
Policy specialists from the J. Craig Venter Institute (JCVI), the University of Virginia, and EMBO released a report that...
OSAKA – Embattled scientist Haruko Obokata has agreed to retract one of two STAP cell research papers from the journal...



