New Jersey Keeps Newborn DNA for 23 Years. Parents Are Suing
By Emily Mullin,
Wired
| 11. 08. 2023
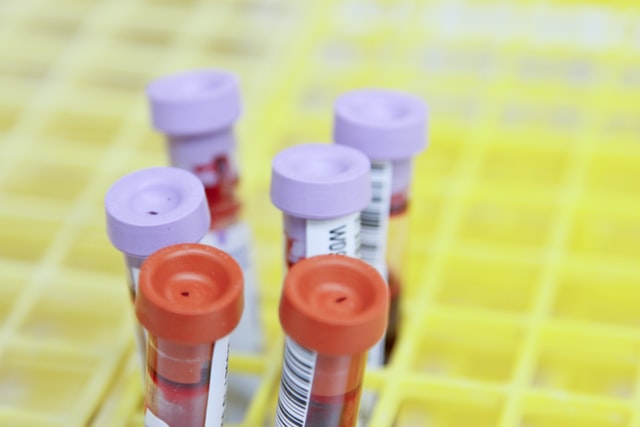
image "Blood Samples" by Daniel Sone from the website of the National Cancer Institute
When Hannah Lovaglio’s children were born, she didn’t think twice about the newborn health screening they received in the hospital. The routine test uses a few drops of blood from a heel prick to test for dozens of potentially fatal or disabling genetic diseases.
“I assumed that this was for my child’s best interest and for the best interest of public health,” says Lovaglio, a pastor in New Jersey. What she didn’t know was that after testing was completed, the leftover blood would be stored by the state for 23 years and could be used for purposes beyond medical testing. In a court case that became public last year, New Jersey police allegedly used a baby’s blood sample to investigate its father for a crime.
Lovaglio has joined a federal class action against the New Jersey Department of Health and its Division of Family Health Services, which oversees the state’s newborn testing program. She and the other plaintiffs are suing the state over its storage practices...
Related Articles
By Scott Solomon, The MIT Press Reader | 02.12.2026
Chris Mason is a man in a hurry.
“Sometimes walking from the subway to the lab takes too long, so I’ll start running,” he told me over breakfast at a bistro near his home in Brooklyn on a crisp...
By Katrina Miller, The New York TImes | 02.05.2026
Joseph Yracheta: The Native Biodata Consortium is the first nonprofit data and sample repository within the geographic bounds and legal jurisdiction of an American Indian nation, on the Cheyenne River Sioux Reservation in Eagle Butte, S.D.
NativeBio participated in a ...
By David Jensen, California Stem Cell Report | 02.10.2026
Touchy issues involving accusations that California’s $12 billion gene and stem cell research agency is pushing aside “good science” in favor of new priorities and preferences will be aired again in late March at a public meeting in Sacramento.
The...
By Lauren Hammer Breslow and Vanessa Smith, Bill of Health | 01.28.2026
On Jan. 24, 2026, the New York Times reported that DNA sequences contributed by children and families to support a federal effort to understand adolescent brain development were later co-opted by other researchers and used to publish “race science”...