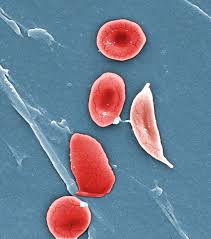
Bluebird suspends studies of sickle cell gene therapy following cancer diagnoses in two more treated patients
By Adam Feuerstein,
STAT
| 02. 16. 2021
Bluebird Bio said Tuesday that it has suspended clinical trials involving its gene therapy for sickle cell disease after receiving reports that two patients treated with the one-time therapy were diagnosed with cancer.
The trials were placed on “temporary suspension” so that Bluebird can investigate the cancer cases to determine if they were caused by the re-engineered HIV virus used to deliver its gene therapy. No such link has been established yet, the company said.
In December 2018, Bluebird disclosed the diagnosis of myelodysplastic syndrome (MDS), a cancer-like disease of the bone marrow, in a sickle cell disease patient who had undergone treatment with its Lentiglobin gene therapy three years beforehand. At that time, Bluebird concluded that the chemotherapy administered to the patient to prepare for the gene therapy was likely the cause of the cancer, based on tests it conducted. The patient subsequently died last July.
The new cancer cases, however, will refocus attention on a possible cancer risk inherent with Bluebird’s gene therapy — and also raise concerns for any gene therapy company that uses re-engineered lentiviruses as...
Related Articles
By Diaa Hadid and Shweta Desai, NPR | 01.29.2026
MUMBRA, India — The afternoon sun shines on the woman in a commuter-town café, highlighting her almond-shaped eyes and pale skin, a look often sought after by couples who need an egg to have a baby.
"I have good eggs,"...
By George Janes, BioNews | 01.12.2026
A heart attack patient has become the first person to be treated in a clinical trial of an experimental gene therapy, which aims to strengthen blood vessels after coronary bypass surgery.
Coronary artery bypass surgery is performed to treat...
By Staff, ScienceDaily | 01.05.2026
Scientists at UNSW Sydney have developed a new form of CRISPR technology that could make gene therapy safer while also resolving a decades-long debate about how genes are switched off. The research shows that small chemical markers attached to DNA
...
Following a long-standing CGS tradition, we present a selection of our favorite Biopolitical Times posts of the past year.
In 2025, we published up to four posts every month, written by 12 authors (staff, consultants and allies), some in collaboration and one simply credited to CGS.
These titles are presented in chronological order, except for three In Memoriam notices, which follow. Many more posts that are worth your time can be found in the archive. Scroll down and “VIEW...