The Direct-to-Consumer Stem Cell Industry in the US
Stem-cell clinics can be found around the world: Mexico, South Korea, the Philippines, China and many other countries. Several have been exposed as scams, and others are suspect, while several high-profile patients (not all) have claimed to have been cured, or at least helped, by them. Two competing storylines have become standard. One is that desperate Americans go abroad for treatments because they were conned; the other is that the FDA is over-cautious and withholding life-saving treatments.
Both narratives assume that few, if any, of these clinics are in the US. That may have been true some years ago: 60 Minutes ran an exposé in 2010 that eventually led to arrests, and another in 2012. Introducing the second one, Scott Pelley made it clear that these were meant as examples, that their team had found “hundreds of credible-looking websites offering stem-cell cures at overseas clinics.”
It seems fair to suggest that up till now many Americans have assumed that the FDA was keeping us safe. That is now in serious question.
Leigh Turner and Paul Knoepfler recently published an important paper in Cell Stem Cell on stem cell clinics in the US. Turner is a University of Minnesota bioethicist and expert on medical tourism; Knoepfler is a stem cell professor at UC Davis who also runs a very well-regarded blog about stem cell research.
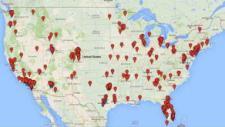
They identified 351 businesses, operating 570 clinics, all over the country (see map above, which is a reduced version of Figure 1 in their article). Some of these clinics may be offering services that do not require FDA approval, but in many cases, Knoepfler explained on his blog,
... there is a strong likelihood that FDA pre-approval would be needed because of issues such as non-homologous use and/or more than minimal manipulation. Such a large industry with unclear regulatory oversight and pre-approval is a big concern overall.
At almost the same time, a horrifying story broke about someone who had traveled to Mexico, China and Argentina for stem cell treatment to help him recover from a stroke. Eventually he developed painful symptoms, which led to surgery that revealed a huge mass of rapidly growing cells in his spine, which were not cancerous, but were "predominantly composed of non host cells,” according to a letter to the New England Journal of Medicine:
Thus, although the lesion may be a considered a neoplasm (i.e., a “new growth”), it could not be assigned to any category of previously described human neoplasm on the basis of the data we gathered.
Stem cell treatments are by no means the only ones that can have unexpected and tragic outcomes, as recent headlines attest. Juno Therapeutics' small clinical trial of an immunotherapy approach to leukemia was abruptly halted last week after it announced that three subjects had died. (Juno later ackowledged a fourth death that occurred last year in a trial of a similar immunotherapy.) Fewer than 20 patients had been enrolled, and “only a minority” of them had the treatment that at first seemed responsible.
The protocol involved taking some of a patient’s own immune cells, performing gene editing on them to target cancerous growths, and then replacing the the rest of the immune cells with the genetically engineered ones. The clinical trial was being conducted under FDA regulations, and the FDA immediately stepped in.
To widespread amazement, it took only two days (the company had expected at least a month) before the FDA agreed that the problem involved a drug interaction and the trial could continue without using the incompatible chemotherapy drug. It’s unusual for the agency to move so fast, but — assuming they were right — it shows that bureaucracy can adapt.
Nevertheless, there are efforts to weaken the system of oversight. Senator Mark Kirk (R, Illinois) has introduced the REGROW Act (Reliable and Effective Growth for Regenerative Health Options that Improve Wellness), which is meant to speed up FDA approval for stem cell treatments. Knoepfler, who called a previous version of the bill “an attack on science-based stem cell trial oversight" remains skeptical:
it over-reaches so much that it would almost certainly do harm to patients and maybe to the stem cell field as a whole.
The Alliance for Regenerative Medicine also opposed the Act, at least in its original form. However, the California Institute of Regenerative Medicine (CIRM) has been campaigning for the FDA to loosen its regulations, even claiming that “patients are dying” because we are “so careful about safety.” CIRM President Randal Mills co-wrote an opinion piece for Fox News with former Senate Majority Leader Bill Frist demanding the the FDA make the approval of “cell therapies” (the word “stem” is not mentioned) easier.
Nature disagrees, in an editorial that references the Turner and Knoepfler article:
FDA should stand firm on stem-cell treatments [headline]
US regulators must regain the upper hand in the approval system. [short]
The pull quote accompanying the editorial is harsh but fair:
"The assumption that these treatments work is at the heart of the problem.”
Previously on Biopolitical Times:
- Hype, Money and Stem Cells
- Selling Stem Cells Honestly
- 60 Minutes Exposes Stem Cell Scams — Again
- 60 Minutes on Stem Cells and Snake Oil
Image via Figure 1 of the paper by Turner and Knoepfler (see above).



